Mol Biol Cell. 2002 December; 13(12): 4279–4295.
doi: 10.1091/mbc.E02-02-0105.
Human
Adipose Tissue Is a Source of Multipotent Stem Cells
Patricia
A. Zuk,*† Min Zhu,* Peter Ashjian,* Daniel
A. De Ugarte,* Jerry I. Huang,* Hiroshi Mizuno,*
Zeni C. Alfonso,‡ John K. Fraser,‡ Prosper Benhaim,*
and Marc H. Hedrick*
*Departments
of Surgery and Orthopedics, Regenerative Bioengineering and Repair Laboratory,
UCLA School of Medicine, Los Angeles, California 90095; and ‡Department
of Medicine and the Jonsson Comprehensive Cancer Center, Division of Hematology
and Oncology, UCLA School of Medicine, Los Angeles, California 90095
PLA cells
were obtained from raw human lipoaspirates and cultured as described in a previous
study (Zuk et al., 2001). Briefly, raw lipoaspirates were washed extensively
with sterile phosphate-buffered saline (PBS) to remove contaminating debris
and red blood cells. Washed aspirates were treated with 0.075% collagenase (type
I; Sigma-Aldrich, St. Louis, MO) in PBS for 30 min at 37°C with gentle agitation.
The collagenase was inactivated with an equal volume of DMEM/10% fetal bovine
serum (FBS) and the infranatant centrifuged for 10 min at low speed. The cellular
pellet was resuspended in DMEM/10% FBS and filtered through a 100-μm mesh
filter to remove debris. The filtrate was centrifuged as detailed above and
plated onto conventional tissue culture plates in control medium (Table 1).
Normal human osteoblasts (NHOst), normal human chondrocytes from the knee (NHCK),
and a population of MSCs from human bone marrow were purchased from Clonetics
(Walkersville, MD) and maintained in commercial medium. The murine 3T3-L1 preadipocyte
cell line (Green
and Meuth, 1974) was obtained from American Type Culture Collection (Manassas,
VA). NHOst, PLA cells, and 3T3-L1 cells were treated with mesenchymal lineage-specific
media as outlined in Table 1.
MSCs were induced using commercial control medium supplemented with the growth
factors outlined in Table 1.
NHOst and NHCK cells were induced using commercially available induction media
(Clonetics).
Antibodies
The antibodies
and commercial sources used in this study are indicated in online Table S1.
Flow Cytometry
PLA cells
and MSCs were cultured in control medium 72 h before analysis. Flow cytometry
with a FACscan argon laser cytometer (BD Biosciences, San Jose, CA) was performed
according to a previous study (Zuk
et al., 2001). Briefly, cells were harvested in 0.25% trypsin/EDTA
and fixed for 30 min in ice-cold 2% formaldehyde. The fixed cells were washed
in flow cytometry buffer (PBS, 2% FBS, 0.2% Tween 20) and incubated for 30 min
in flow cytometry buffer containing fluorescein isothiocyanate-conjugated monoclonal
antibodies to SH3, STRO-1, and the following CD antigens: 13, 14, 16, 31, 34,
44, 45, 49d, 56, 62e, 71, 90, 104, 105, and 106. PLA cells and MSCs were stained
with a phycoerythrin-conjugated nonspecific IgG to assess background fluorescence.
Indirect
Immunofluorescence.
PLA cells
and MSCs were processed as described previously (Zuk
et al., 2001) by using monoclonal antibodies to specific CD markers
and lineage-specific proteins (online Table S1).
Histology
and Immunohistochemistry.
Differentiated
PLA cells and clones were processed as described previously (Zuk
et al., 2001) by using the following histological assays: alkaline
phosphatase (AP) (osteogenesis), Oil Red O (adipogenesis), and Alcian blue (AB)
(chondrogenesis). Chondrogenic PLA cells and clones were examined for collagen
type 2 (CNII), keratan sulfate, and chondroitin-4-sulfate expression by immunohistochemistry
as described previously (Zuk
et al., 2001). Neurogenic PLA cells and clones were examined by immunohistochemistry
for the expression of neural-specific proteins.
Spectrophotometric
Assays
AP.
Triplicate
samples of PLA cells were differentiated in osteogenic medium (OM) for up to
6 wk. Cells were washed with PBS, harvested, and AP enzyme activity was assayed
using a commercial AP enzyme kit according to the method of Beresford
et al. (1986). AP activity was expressed as nanomoles of p-nitrophenol
produced per minute per microgram of protein. Differentiated MSCs were assayed
as a positive control, whereas non-induced PLA cells were assayed as a negative
control. Values are expressed as the mean ± SD. A Student's t test (paired)
was performed to determine statistical significance between induced and control
samples.
Characterization
of MSCs has been performed using the expression of cell-specific proteins and
CD markers (Bruder
et al., 1998b; Conget
and Minguell, 1999; Pittenger
et al., 1999). Like MSCs, PLA cells expressed CD29, CD44, CD71, CD90,
CD105/SH2, and SH3 and were absent for CD31, CD34, and CD45 expression (online
Figure S1). Moreover, flow cytometry on PLA cells confirmed the expression of
CD13, whereas no expression of CD14, 16, 56, 62e, or 104 was detected (Table
2).
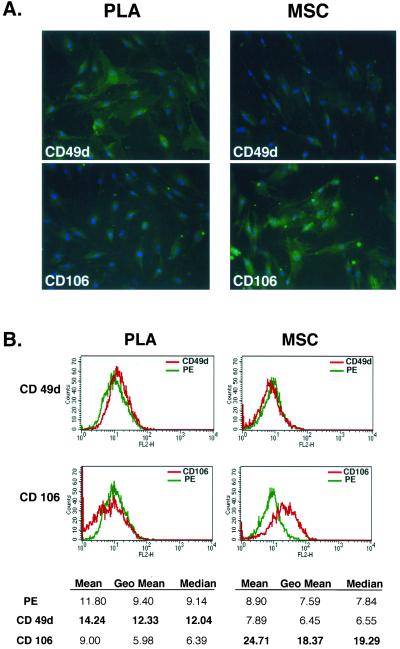
These results demonstrate that similar CD complements are expressed on both
PLA cells and MSCs. However, distinctions in two CD markers were observed: PLA
cells were positive for CD49d and negative for CD106, whereas the opposite was
observed on MSCs. Expression of CD106 has been confirmed in the bone marrow
stroma and, specifically, MSCs (Levesque
et al., 2001) where it is functionally associated with hematopoiesis.
The lack of CD106 on PLA cells is consistent with the localization of these
cells to a non-hematopoietic tissue.
PLA Cells Differentiate into Bone, Fat, Cartilage, and Muscle: Multiple Mesodermal Lineage Capacity
As suggested
in a previous study (Zuk
et al., 2001), PLA cells seem to possess the capacity to differentiate
into multiple mesodermal lineages, including bone, fat, and cartilage. This
observation has led us to speculate that adipose tissue may be a source of mesodermal
stem cells. The current study supports this hypothesis, characterizing the metabolic
activity of several mesodermal lineages, in addition to confirming the expression
of multiple lineage-specific genes and proteins.

Figure
1.
PLA cells
express a unique set of CD markers. (A) PLA cells and MSCs were processed by
immunofluorescence for expression of multiple CD antigens (green). Cells were
costained with 4,6-diamidino-2-phenylindole to visualize nuclei (blue) and the
fluorescent images combined. The differential expression of CD49d and CD106
between PLA cells and MSCs is shown (Figure S1 for remaining CD antigens). (B)
Flow cytometric analysis on PLA cells and MSCs for the expression of CD49d and
CD106 was performed (red). Cells stained with a fluorochrome-conjugated nonspecific
IgG were examined as a control (γPE, green). The geometric mean and median
values for CD49d and Cd106 are shown below. Significant differences are shown
in bold.
Mol Biol
Cell. 2002 December; 13(12): 4279–4295.
Table
2.
Flow cytometric
analysis of CD marker expression on non-induced PLA cells
|
CD
Antigen |
Geometric
Mean |
|
CD13 |
148.88 |
|
CD14 |
2.43 |
|
CD16 |
2.38 |
|
CD31 |
2.22 |
|
CD34 |
3.55 |
|
CD44 |
16.92 |
|
CD45 |
2.52 |
|
CD49d |
14.99 |
|
CD56 |
2.66 |
|
CD62E |
2.30 |
|
CD71 |
3.76 |
|
CD90 |
25.96 |
|
CD104 |
2.31 |
|
CD105 |
8.39 |
|
CD106 |
2.45 |
|
SH3 |
8.95 |
|
STRO-1 |
31.26 |
|
−ve |
2.59 |
Mol Biol
Cell. 2002 December; 13(12): 4279–4295.
From: http://www.rndsystems.com/asp/g_sitebuilder.asp?bodyId=472
STRO-1: The
murine IgM monoclonal Ab STRO-1, produced from an immunization with a population
of human CD34+ bone marrow cells, can identify a cell surface antigen
expressed by stromal elements in human bone marrow.55 From bone marrow
cells, the frequency of fibroblast colony-forming cells (CFU-F) is enriched
approximately 100-fold in the STRO-1+/Glycophorin A- population than
in the STRO-1+/Glycophorin A+ population.55
A STRO-1+ enriched subset of marrow cells is capable of differentiating
into multiple mesenchymal lineages including hematopoiesis-supportive stromal
cells with a vascular smooth muscle-like phenotype, adipocytes, osteoblasts
and chondrocytes.56-59 STRO-1 is a valuable Ab for the identification,
isolation and functional characterization of human bone marrow stromal cell
precursors, which are quite distinct from those of primitive HSCs.
From: http://www.sciencenews.org/articles/20040320/bob8.asp
A dangerous
cell
One of
the main issues regarding cancer stem cells is whether they're normal stem cells
gone awry or differentiated cells that have acquired stem cell characteristics.
The former scenario appeals to most scientists, although they acknowledge it's
largely unproved.
Because
it can replicate endlessly, a normal stem cell is a "very dangerous cell" that's
poised on the edge of becoming cancerous, says Dick. The potentially endless
reproduction of a stem cell also allows enough time for cancer-promoting mutations
to accumulate in such a cell, he explains.
The cancer–stem-cell
hypothesis could explain why many cancers are resistant to radiation and drugs.
Normal stem cells are unusually hardy and possess molecular pumps similar to
the ones that some cancer cells use to flush out chemotherapy agents, notes
Kornblum.
The discovery
of cancer stem cells is forcing scientists to reconsider how they look for tumor-fighting
drugs. "Everyone has been concentrating on proliferation," says Clark. Traditionally,
researchers screen for compounds that kill dividing tumor cells, but stem cells
are often quiescent, only occasionally spawning progeny that then rapidly proliferate.
"The biology
of the tumor you see may not be the same as the biology of the stem cell. You're
never going to cure someone unless you hit the stem cell," says Matsui.
Scientists
battling leukemia, the disease in which a cancer stem cell was first isolated,
have been focusing on this new target for a few years, says Dick. As one example,
he points to a 2002 study in which Craig T. Jordan of the University of Kentucky
Medical Center in Lexington and his colleagues identified compounds that specifically
kill leukemia stem cells derived from patients.
The research
on cancer stem cells also threatens to upend thinking on how cancers spread,
or metastasize. Conventional theories hold that metastasis is an evolutionary
process in which a small number of cells within a primary tumor gradually accumulate
the genetic mutations that enable them to spread to other tissues and establish
new tumors. An alternative model now being put forth is that many cells in a
primary tumor spread in the body, but a second tumor arises only when a rare
stem cell reaches a new site.
Scientists
have proposed that identifying cancer stem cells from various types of tumors
will help them isolate the long-sought normal stem cells in tissues such as
the prostate gland and the breast. "Tracing back from the tumor to that cell
population will allow us to identify these critical cells in normal tissue,"
says Jacks, who is a Howard Hughes Medical Institute investigator at MIT.
Bo
Zheng, M.D.
Postdoctoral Research Fellow
University of Pittsburgh
bozheng@pitt.edu
bozheng72@yahoo.com
Tel: 412-692-3239(O)
Fax: 412-692-7095
In recent years, scientists
have discovered a wide array of stem cells that have unique capabilities to
self-renew, grow indefinitely, and differentiate of develop into multiple types
of cells and tissues. My works focus the isolation of adult stem cells from
a variety of tissues and organs. In the present study, we characterized a population
of potential adipose-derived adult stem (ADAS) cells isolated from the visceral
fat of the abdominal cavity of C57BL/10J mice. We used flow cytometry to examine
the marker profile (CD34 and Sca-1) of these cells. The isolated cells were
CD45-negative, which excludes any possible contamination by hematopoietic cells,
and were partially positive for Sca-1 (38%) and CD34 (7%), two stem cell markers.
After induction in conditioned medium, the ADAS cells differentiated into adipogenic,
osteogenic, chondrogenic, and myogenic lineages. This finding supports previous
reports that indicate the existence of multipotent stem cells in a variety of
adult tissues. This finding is consistent with the cells isolated from human
and rat fat tissue. These findings suggest that adipose tissue may be a novel,
easily accessible, and replenishable source of pluripotent stem cells suitable
for cellular therapies.
International
Fat Applied Technology Society:
One pint of liposuctioned fat or one pound of whole fat removed in a
tummy tuck, for example, can yield up to 200 million stem cells, which in culture
can be expanded by 10 times over the course of two weeks.
Although
he offers cell-harvesting services to all of his patients, Dr. Ersek says, "Not
that many are interested because they've never heard of it. New ideas take awhile
to grab hold, even really good ones. So I decided to store my own stem cells.
And it occurred to me that in order to convince my patients that (the procedure)
is very safe and simple, I decided to harvest the stem cells myself. So I put
in some local anesthesia and performed the procedure entirely awake, just using
Xylocaine with epinephrine. This was not a liposuction procedure for changing
my size. We took out about one pound of fat just for the stem cell purpose."
StemSource
later processed Dr. Ersek's tissue down to 150 cc of usable material, which
yielded 4 million viable stem cells. The company stores such materials at minus
320 degrees Fahrenheit, charging patients $1,675 for five years' storage (or
$600 initially plus $175 annually after the first year). For more
information about cell preservation and banking contact:
MacroPore Biosurgery, Elizabeth Scarbrough
Vice President Marketing and Development – Biologics
740 Top
Gun, San Diego California 92121
Phone: 858-458-0900, Toll Free: 877-470-8000
From: http://www.miltenyibiotec.com/macs/products/fluorochrome/cd133.htm
 CD133+
cells budding from the adherent cell surface by asymetric cell division. Cells
were stained with CD133/1 (AC133)-PE.
CD133+
cells budding from the adherent cell surface by asymetric cell division. Cells
were stained with CD133/1 (AC133)-PE.
CD133
is expressed on immature hematopoietic stem and progenitor cells, and is not
found on mature blood cells. In contrast to the CD34 antigen, CD133 is not expressed
by late progenitors, such as pre-B cells, CFU-E, and CFU-G.
The antibodies specific for the CD133 antigen (clones AC133, 293C3 and AC141)
stain 35–75% of the CD34+ population including CD34bright,
CD38neg/dim, HLA-DR–, CD90+ and CD117+
cells. Moreover, a small population of CD133+CD34– cells
with long-term repopulating potential was identified.
Functional studies on MACS-isolated CD133+ cells confirmed that CD133
is expressed on long-term culture initiating and long-term repopulating hematopoietic
stem cells.
Further, certain acute myeloid leukemias have been reported to be positive for
CD34, but negative for CD133. Therefore, CD133 selected cells have already been
used for autologous transplantation ALL.
HomePage Equipment Procedures Retrieval Extraction
Updated: Monday, December 27, 2004 0:43 AM